Factory Supply Food Additives Beta Cyclodextrin 7585-39-9
Basic Info
| Model NO. | HMJ-Beta Cyclodextrin |
| Powder | Yes |
| Customized | Non-Customized |
| Certification | GMP, HSE, ISO 9001, USP, BP |
| Suitable for | Elderly, Children, Adult |
| State | Powder |
| Purity | >99% |
| Color | White |
| Mf | C42h70o35 |
| Einecs | 231-493-2 |
| Molecular Weight | 1134.99 |
| Test Method | HPLC |
| CAS | 7585-39-9 |
| Shelf Life | 2 Years |
| Grade | Pharmaceutical Grade |
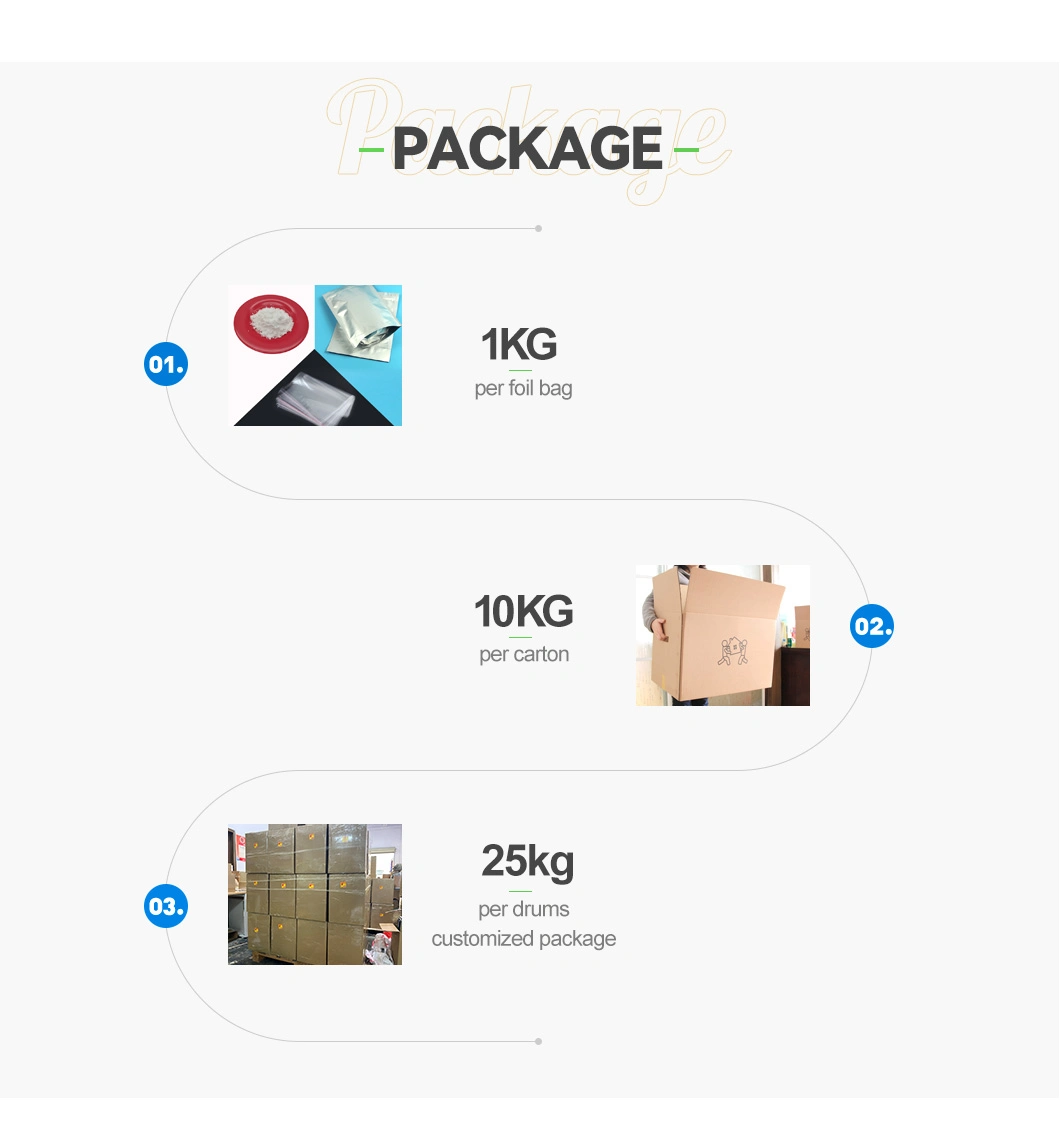
| Transport Package | Ziplock Foil Bag. Drum |
| Specification | 1kg Per Foil Bag, 10 Bags Per Carton, 25 Kg Per Dr |
| Trademark | HMJ |
| Origin | China |
| Production Capacity | 200kg/Month |
Product Description
Factory Supply Food Additives Beta Cyclodextrin 7585-39-9
Product Description
| Product Name | Factory Supply Food Additives Beta Cyclodextrin 7585-39-9 |
| Appearance | White powder |
| Molecular formula | C42H70O35 |
| Molecular weight | 1134.99 |
| Keywords | Beta Cyclodextrin , Material Beta Cyclodextrin ; Pure Beta Cyclodextrin |
| Shelf Life | 24 months when properly stored |
| Storage | Keep in a cool, dry, dark location |
What is Beta Cyclodextrin
The production of cyclodextrins is relatively simple and involves treatment of ordinary starch with a set of easily available enzymes. Commonly cyclodextrin glycosyltransferase (CGTase) is employed along with α -amylase. First starch is liquified either by heat treatment or using α -amylase, then CGTase is added for the enzymatic conversion. CGTases can synthesize all forms of cyclodextrins, thus the product of the conversion results in a mixture of the three main types of cyclic molecules, in ratios that are strictly dependent on the enzyme used: Each CGTase has its own characteristic α : β : γ Synthesis ratio. Purification of the three types of cyclodextrins takes advantage of the different water solubility of the molecules: β -CD which is very poorly water soluble (18.5 g/l or 16.3mM) (at 25C? ? ? ) can be easily retrieved through crystallization while the more soluble α - and γ -CDs (145 and 232 g/l respectively) are usually purified by means of expensive and time consuming chromatography techniques. As an alternative a "complexing agent" can be added during the enzymatic conversion step: Such agents (usually organic solvents, ethanol) form a complex with the desired cyclodextrin which subsequently precipitates. The complex formation drives the conversion of starch towards the synthesis of the precipitated cyclodextrin, thus enriching its content in the final mixture of products. The precipitated cyclodextrin is easily retrieved by centrifugation and is later separated from the complexing agent.
Function of Beta Cyclodextrin
Crystal structure of a rotaxane with an α -cyclodextrin macrocycle. Cyclodextrins are able to form host-guest complexes with hydrophobic molecules given the unique nature imparted by their structure. As a result these molecules have found a number of applications in a wide range of fields. Other than the above mentioned pharmaceutical applications for drug release, cyclodextrins can be employed in environmental protection: These molecules can effectively immobilise inside their rings toxic compounds, like trichloroethane or heavy metals, or can form complexes with stable substances, like trichlorfon (an organophosphorus insecticide) or sewage sludge, enhancing their decomposition.In the food industry cyclodextrins are employed for the preparation of cholesterol free products: The bulky and hydrophobic cholesterol molecule is easily lodged inside cyclodextrin rings that are then removed. Other food applications further include the ability to stabilize volatile or unstable compounds and the reduction of unwanted tastes and odour. Reportedly cyclodextrins are used in alcohol powder, a powder for mixing alcoholic drinks.The strong ability of complexing fragrances can also be used for another purpose: First dry, solid cyclodextrin microparticles are exposed to a controlled contact with fumes of active compounds, then they are added to fabric or paper products. Such devices are capable of releasing fragrances during ironing or when heated by human body. Such a device commonly used is a typical 'dryer sheet'. The heat from a clothes dryer releases the fragrance into the clothing.The ability of cyclodextrins to form complexes with hydrophobic molecules has LED to their usage in supramolecular chemistry. In particular they have been used to synthesize certain mechanically-interlocked molecular architectures, such as rotaxanes and catenanes, by reacting the ends of the threaded guest.The application of cyclodextrin as supramolecular carrier are also possible in organometallic reaction. The mechanism of action probably take place in the interfacial region (see L. Leclercq et al. ). Wipff are also demonstrated by computational study that the reaction occur in the interfacial layer. The application of cyclodextrins as supramolecular carrier is possible in various organomettalic catalysis.
Detailed Photos Related Product